I. Introduction
Adipose tissue plays a central role in the interplay between nutrition, energy balance, and human health. There are 2 types of adipose tissue, white and brown. White adipose tissue (WAT) stores energy, whereas brown adipose tissue (BAT) dissipates it. Overnutrition and/or physical inactivity result in an excess of WAT, the hallmark of obesity. In contrast, BAT is thermogenic, a property conferred by the presence of a unique protein, uncoupling protein 1 (UCP1). Located in the inner mitochondrial membrane, UCP1 uncouples mitochondrial respiration, releasing energy as heat. This unique property protects animals from hypothermia (1).
The traditional belief that BAT exists only in infants but not in adults has resulted in a paucity of research in humans. However, the discovery of fat with high metabolic activity in adults by functional imaging using positron emission tomography (PET) brought about a resurgence in research interest on BAT identity, abundance, prevalence, regulation, and significance in humans (2ñ9).
This review will cover 1) the characteristics and ontogeny of BAT, 2) its prevalence and regulation, 3) metabolic relevance, 4) the potential roles of BAT in health and diseases, and 5) the avenues for therapeutic targeting of BAT in obesity. These questions will be discussed on the background of known biology of BAT in rodents.
II. A Historical Perspective
Understanding the biology of a body organ requires knowledge of location, morphology, regulation, and function. In contrast to other classic metabolic organs, such as skeletal muscle, WAT, and liver, the biology of BAT in humans has remained elusive since Gessner first described its presence in hibernators in 1551. The 1960s heralded a golden era of human BAT research that withered in the late 1980s with the view that significant deposits of BAT did not persist beyond childhood. In the last decade, unequivocal evidence of BAT in adult humans has led to resurgence in global research interest. Table 1 summarizes major developments in human BAT research.
Data table
Table 1. Timeline and Summary of Major Developments in Human BAT Research
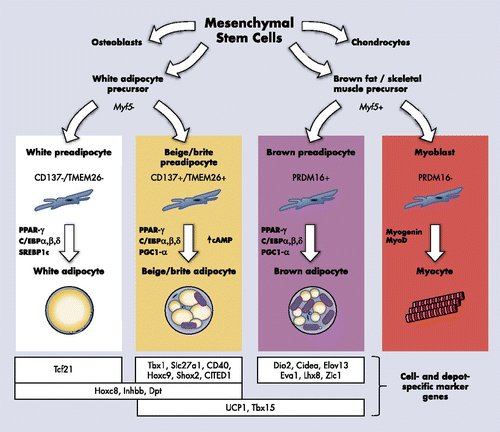
At the beginning of the last century, anatomists described similarities between fat masses located in the dorsal and cervical region of human fetuses and fat depots in the interscapular area of hibernating mammals (10ñ12). It was, however, not until the 1960s that BAT was ascribed a regulatory role in thermogenenesis (13ñ18). It was proposed that BAT was a heat-producing tissue in small mammals and human infants, defending newborns from hypothermia. BAT is histologically and functionally distinct from WAT, and the presence of the facultative proton transporter UCP1 confers upon it the unique ability to generate heat through respiratory uncoupling (19).
The thermogenic properties of BAT originally were of interest only to a few scientists studying hibernating animals. Serial publications revealed a 6-fold increase in heat production from BAT after cold acclimatization in rodents, dissipating heat to the body via dense vascularization juxtaposing deep viscera (13, 20ñ22). The striking thermogenic capacity of BAT led some researchers to regard it as an electric blanket for animals in the cold (23). Because temperature changes are cues to food availability in nature, BAT studies were extended to investigating response to nutrient variations. In the 1970s, Rothwell and Stock (24) observed near identical morphological changes in BAT between cold-exposed and high-fat diet-fed animals. Heat production in BAT during cold exposure corresponded closely to that after high-fat feeding. The strong association between diet-induced thermogenesis (DIT) and cold-induced thermogenesis (CIT) in animals led to the proposal that BAT played a major role in both. Meanwhile, from human cadaveric studies, Heaton (25) found that BAT persisted up to the eighth decade of life. These findings led to the hypothesis that BAT failure could contribute to development of obesity in adult humans (26ñ29), resulting in a tripling of BAT publications between 1980 and 1982.
During this early phase of human BAT research, investigations were restricted to examining depots around the adrenal bed, a location accessible during elective abdominal surgery. This approach overlooked BAT in extra-abdominal locations, underestimating its abundance and distribution in adults. The scientific consensus at the time did not support a definite metabolic role in energy homeostasis in adult humans (30, 31), with Rothwell and Stock raising the doubt of ìWhither brown fat?î (32). It was recognized there were major difficulties identifying BAT depots in humans and that the view would ìcontinue to be controversial until a method for quantitative noninvasive measurement of total BAT thermogenesis is developedî (30, 33).
It took another 2 decades for such noninvasive methods to become available. PET scanning technology has ushered in a new era of metabolic imaging, catalyzing the resurgence in BAT research. The rebirth of human BAT research interest has been viewed as a renaissance in metabolic medicine (34). The research questions in human BAT are the same as those posed in the 1980s; however, the field has been enriched by advances and insights from animal studies in the intervening years. This review will integrate new knowledge from animal studies into an appraisal of its physiological significance in humans.